-
Our Services
- New Industrialisation
-
Smart Manufacturing
- Smart Manufacturing
-
IIOT
- IIOT
- Industrie 4.0 - Smart Enterprise
- Product Lifecycle Management Consultancy
- Intelligent Automatic Warehousing and Logistics
- Real-Time Manufacturing Tracking System
- Knowledge Based Engineering & CAD Solution
- Location Based Services and Location Analytics
- The HATCH
- Hong Kong Industrial Drone Technology Centre
-
AI & Robotics
- AI & Robotics
- AI Sheet Metal Surface Defect Detection Technology
- Product Innovation and Design Management
- Robotics Application
- Automation Feasibility Study
- 3D Automatic Processing
- Application and Research on Vision and Ultrasonic Inspection Assisted by Artificial Intelligence and Robotics
- Customized Intelligent and Cognition Automation Machine & System Development
- Autonomous Air-ground Cooperative Tunnel Inspector
- AR-Empowered Robot Control System (ARERC System)
- IoT-enabled Smart Toilet Bowl Cleaning System
- Novel Materials
-
Advanced Manufacturing Technology
- Advanced Manufacturing Technology
- 3D Scanning and Reverse Engineering Service
- Flexible Metallic Fiber Physical Porous Part Fabrication Technology
- Advanced Mould Cooling Technology and CAE Conformal Cooling Analysis
- Gas Atomisation Technology
- Dual Laser Metal Polishing Technology
- Advanced Additive Manufacturing, 3D Printing Technology, and Direct Manufacturing
- Diffusion Bonding Technology
- Electrically-Assisted Free Forming (EAFF) Technology for Customisation of Sheet Metal Parts
- Plastic Process and Machinery Technology
- Fashion and Garment Technology
- Computer Aided Technology (CAx)
- Watch Assembly Automation Technology
-
Digital Transformation
- Digital Transformation
- HKPC Digital DIY Portal
- Digital Transformation Support
- 「FitEasy」Virtual Fitting Technology - For People with Disability
- Smart Solution
- Research and Analytics
- Strategic IT Management
- Embedded Software System
- New Media and Learning Technology Development
- IT Industry Support
- DevOps Maturity Assessment and Consultancy Service
- Software Testing Automation Consultancy Service
- Blockchain Consulting Service
- Extended Reality (XR) technology and consultancy service
-
Cyber Security
- Cyber Security
- Cyber Security
- Cybers Security-by-design, Privacy and Compliance-by-default
- Design & Architecture
- Train & Develop
- Offensive Security
- Intelligent Security
- Defensive Security
- Intelligent Hardening
- Internet of Things (IoT) & Operational Technology (OT) Cyber Security Testing
- Phishing Defence Services
- Cyber Security Assessment & Audit
- Cyber Security Consultancy for i4.0 & e4.0
-
Green & Smart Living
- Green & Smart Living
- Green Technology
-
HealthTech and Chinese Medicine
- HealthTech and Chinese Medicine
- R&D service and Functional Investigation on Chinese Medicine, Health Food & Wellness product
- Compliance Consultation Service for Chinese Medicine, Pharmaceutical, Health Food and Medical Device Industries
- Manufacturing Enhancement - Automatic Intelligent System for Production and Packaging in Chinese Medicine, Pharmaceutical and Health Food Industries
- Assisting funding application for local medicine and health industrial associations
- “The Cradle” Services for Health Tech and TCM Industry
- Food Technology
- Smart Living
-
Corporate Sustainability
- Corporate Sustainability
- ESG and Sustainability Services
- Manufacturing Technology (Tooling, Metals & Plastics) Recognition of Prior Learning (RPL) Mechanism
- Market Research and Analytics
- Business Innovation
- Sustainability-related standards and guidance
- Organisation Innovation Capability Development
- District Innovation
- Customer Service Assessment
- Intellectual Property (IP) Protection and Management
- Support to Creative Industries
- Manufacturing Standards Consultancy Service
- Production Capacity Optimisation
- Cost of Quality
- FutureSkills
- SME Support
- Funding
- Testing & Standards
-
Support & Resource
- Technology Transfer
-
Support Centres
- Support Centres
- The Cradle – Go Global Service Centre
- Life & Health Tech Hall
- HKPC-HP 3D Printing Technology Centre
- Future Manufacturing Hall
- Celesphere
- Inno Space
- The HATCH
- Intellectual Property Services Centre
- Advanced Electronics Processing Technology Centre
- Green Living Laboratory
- Reliability Testing Centre
- Electromagnetic Compatibility Centre
- Plastics Technology Centre
- Smart Wearables, Watch & Clock Technology Centre
- Conformal Cooling Technology Centre
- Hong Kong Digital Testing Hub
- Hong Kong Industrial Drone Technology Centre
- Aqua Research Laboratory
- Advanced Materials and Intelligent Manufacturing Centre
- Hong Kong Joint Research Lab for Applications of Intelligent Automation Technology
- Future FoodTech Lab
- HKUST-HKPC Joint Research Lab for Industrial AI and Robotics
- Hong Kong Industrial Artificial Intelligence & Robotics Centre (FLAIR)
- Testing & Standards
- HKPC Spotlights
- About US
-
 LANGUAGE
LANGUAGE
IIOT
- Industrie 4.0 - Smart Enterprise
- Product Lifecycle Management Consultancy
- Intelligent Automatic Warehousing and Logistics
- Real-Time Manufacturing Tracking System
- Knowledge Based Engineering & CAD Solution
- Location Based Services and Location Analytics
- The HATCH
- Hong Kong Industrial Drone Technology Centre
AI & Robotics
- AI Sheet Metal Surface Defect Detection Technology
- Product Innovation and Design Management
- Robotics Application
- Automation Feasibility Study
- 3D Automatic Processing
- Application and Research on Vision and Ultrasonic Inspection Assisted by Artificial Intelligence and Robotics
- Customized Intelligent and Cognition Automation Machine & System Development
- Autonomous Air-ground Cooperative Tunnel Inspector
- AR-Empowered Robot Control System (ARERC System)
- IoT-enabled Smart Toilet Bowl Cleaning System
Advanced Manufacturing Technology
- 3D Scanning and Reverse Engineering Service
- Flexible Metallic Fiber Physical Porous Part Fabrication Technology
- Advanced Mould Cooling Technology and CAE Conformal Cooling Analysis
- Gas Atomisation Technology
- Dual Laser Metal Polishing Technology
- Advanced Additive Manufacturing, 3D Printing Technology, and Direct Manufacturing
- Diffusion Bonding Technology
- Electrically-Assisted Free Forming (EAFF) Technology for Customisation of Sheet Metal Parts
- Plastic Process and Machinery Technology
- Fashion and Garment Technology
- Computer Aided Technology (CAx)
- Watch Assembly Automation Technology
Digital Transformation
- HKPC Digital DIY Portal
- Digital Transformation Support
- 「FitEasy」Virtual Fitting Technology - For People with Disability
- Smart Solution
- Research and Analytics
- Strategic IT Management
- Embedded Software System
- New Media and Learning Technology Development
- IT Industry Support
- DevOps Maturity Assessment and Consultancy Service
- Software Testing Automation Consultancy Service
- Blockchain Consulting Service
- Extended Reality (XR) technology and consultancy service
Cyber Security
- Cyber Security
- Cybers Security-by-design, Privacy and Compliance-by-default
- Design & Architecture
- Train & Develop
- Offensive Security
- Intelligent Security
- Defensive Security
- Intelligent Hardening
- Internet of Things (IoT) & Operational Technology (OT) Cyber Security Testing
- Phishing Defence Services
- Cyber Security Assessment & Audit
- Cyber Security Consultancy for i4.0 & e4.0
HealthTech and Chinese Medicine
- R&D service and Functional Investigation on Chinese Medicine, Health Food & Wellness product
- Compliance Consultation Service for Chinese Medicine, Pharmaceutical, Health Food and Medical Device Industries
- Manufacturing Enhancement - Automatic Intelligent System for Production and Packaging in Chinese Medicine, Pharmaceutical and Health Food Industries
- Assisting funding application for local medicine and health industrial associations
- “The Cradle” Services for Health Tech and TCM Industry
Corporate Sustainability
- ESG and Sustainability Services
- Manufacturing Technology (Tooling, Metals & Plastics) Recognition of Prior Learning (RPL) Mechanism
- Market Research and Analytics
- Business Innovation
- Sustainability-related standards and guidance
- Organisation Innovation Capability Development
- District Innovation
- Customer Service Assessment
- Intellectual Property (IP) Protection and Management
- Support to Creative Industries
- Manufacturing Standards Consultancy Service
- Production Capacity Optimisation
- Cost of Quality
Support Centres
- The Cradle – Go Global Service Centre
- Life & Health Tech Hall
- HKPC-HP 3D Printing Technology Centre
- Future Manufacturing Hall
- Celesphere
- Inno Space
- The HATCH
- Intellectual Property Services Centre
- Advanced Electronics Processing Technology Centre
- Green Living Laboratory
- Reliability Testing Centre
- Electromagnetic Compatibility Centre
- Plastics Technology Centre
- Smart Wearables, Watch & Clock Technology Centre
- Conformal Cooling Technology Centre
- Hong Kong Digital Testing Hub
- Hong Kong Industrial Drone Technology Centre
- Aqua Research Laboratory
- Advanced Materials and Intelligent Manufacturing Centre
- Hong Kong Joint Research Lab for Applications of Intelligent Automation Technology
- Future FoodTech Lab
- HKUST-HKPC Joint Research Lab for Industrial AI and Robotics
- Hong Kong Industrial Artificial Intelligence & Robotics Centre (FLAIR)
- R&D service and Functional Investigation on Chinese Medicine, Health Food & Wellness product
- Compliance Consultation Service for Chinese Medicine, Pharmaceutical, Health Food and Medical Device Industries
- Manufacturing Enhancement - Automatic Intelligent System for Production and Packaging in Chinese Medicine, Pharmaceutical and Health Food Industries
- Assisting funding application for local medicine and health industrial associations
- “The Cradle” Services for Health Tech and TCM Industry
“The Cradle” Services for Health Tech and TCM Industry
Life sciences and Traditional Chinese Medicine (TCM) technology are key industries in Hong Kong's Innovation and Technology Development Blueprint, and they also form a core part of China's 14th Five-Year Plan, creating global expansion opportunities for Hong Kong's innovation sector. HKPC's HealthTech and TCM unit, New Industrialisation Division, provides comprehensive “Cradle” support, including market research, product innovation and development, regulatory compliance and product registration, quality assurance, GMP facility design, production line setup, overseas certification, and funding applications. We customise global expansion plans for each enterprise, tailored to its specific needs, resources, and development stage.
Market Research and Product Innovation & Development
HKPC promotes technology upgrades, leveraging industrial internet and common platforms to foster innovation and digital transformation in Healthtech and TCM products, enhancing product quality and production efficiency, and developing new productivity strategies tailored to their needs. Our service includes, but is not limited to: Providing advice on the latest discoveries of active ingredients and frontier topics in life science research fields.
- Providing recommendations on the latest discoveries and frontier topics in the research of active ingredients
- Developing innovative products, scientific TCM, or life science-related series products.
- Piloting current and future products’ market entry, conducting comparative analysis of similar products in overseas markets.
- Aligning product development timeline and standards to registration requirements.
Product Innovation & Development

Testing services for products or drugs
HKPC leverages its extensive local and international cooperation network to develop internationally compliant products for enterprises. Our service includes, but is not limited to:
- Conducting quality and efficacy tests on formulas or products according to local government requirements or international standards, including but not limited to physiochemical properties, quality standards, testing methods and reports, stability test reports, pharmacological and toxicological research, etc.
- Selecting appropriate cell and/or animal models for mechanism studies of drugs/health-promoting effects.
Study on the effectiveness and quality of drugs or products

Ultraviolet Fluorescence-based zebra fish model
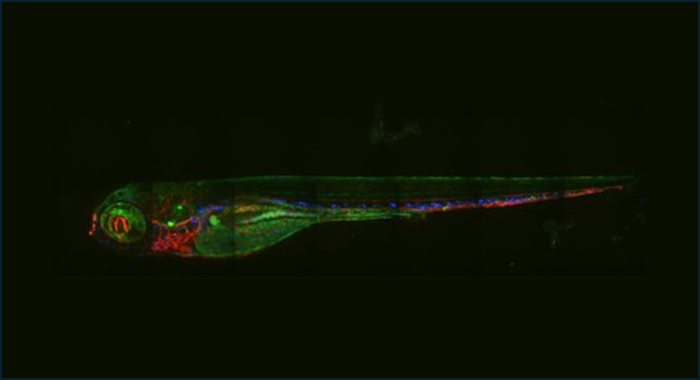
In vivo green fluorescent (FITC) protein expression
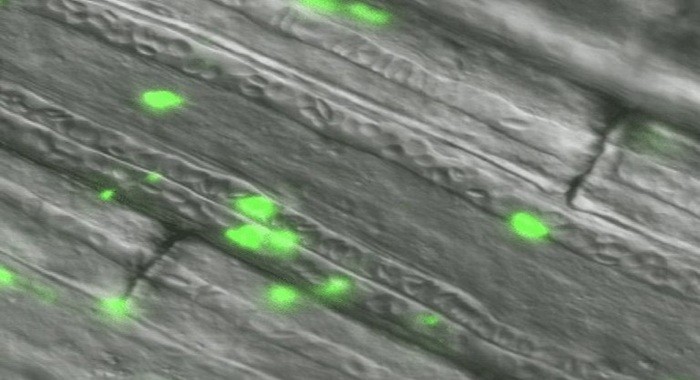
Regulation Compliance and Product Registration
HKPC provides compliance consultation on the local and overseas markets. Our service includes, but is not limited to:
- Offering regulatory compliance consultation for international market entry products.
- Handling product or drug registration applications, devising optimal registration strategies.
- Providing clinical sample production, analysis method development, and stability studies.
- Responding to regulatory inquiries and follow-up supplementary documentation.
Drug registration

GMP Plant Design
The HealthTech and TCM unit under HKPC's New Industrialisation Division possesses extensive knowledge and experience in production quality management (GMP) and related certifications, making adjustments based on User Requirements Specification (URS). Our service includes, but is not limited to:
- Conducting preliminary assessment and planning layout for GMP certification.
- Conducting GMP gap analysis for new/renovated factories/workshops.
- Designing and installing clean rooms for production and packaging needs.
- Designing and installing heating, ventilation, air conditioning systems (HVAC), compressed air, and pure water systems.
- Overseeing the validation plan for the cleanroom and the three major systems
- Auditing GMP certification equipment suppliers and providing validation testing for clean rooms and the three major systems.
- Preparing Approval in Principle (AIP), including reviewing Plant Master File (PMF) and Standard Operating Procedure (SoP) documentation.
- Dealing with health department follow-up enquiries and rectifying required documents according to guidelines.
Plant Design
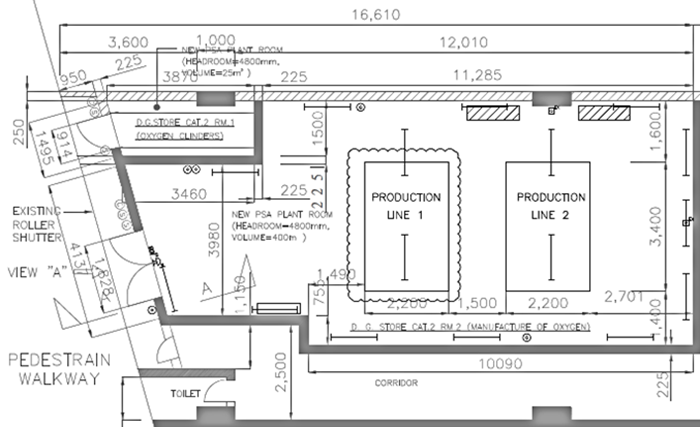
Production Line or System Construction
HKPC designs and builds artificial intelligence production lines or systems for enterprises, and has been awarded multiple local and international invention awards, which brings Hong Kong technology to the world. In one case study for a TCM enterprise, HKPC proposed AI production under strict GMP production quality management, improving internal and external packaging processes of TCM bottles, increasing productivity by 11 times and production volume by 100%. Our service includes, but is not limited to:
- Providing an AI automated system design that complies with GMP production quality management standards
- Using modularised design and a smart programming production system for the packaging of different sizes and textures
- Employing machine vision systems to ensure high-quality standards of the appearance and the conformation processes, including packaging, instruction sheets, sealing, and anti-counterfeit labels
- Incorporating a real-time data collection system (production, equipment, quality, and energy data) to monitor automated system performance, in preparation for the establishment of the Industrial Internet of Things (IIoT)
- Utilising an easy-to-use Human Machine Interface (HMI)
- Employing industrial robots with other devices for high-speed processing, transportation of various irregular materials and precise allocation of tiny bottles into thermoform packs without disrupting the surrounding area
Drug packaging and assembling robotic arm system
Intelligent visual packaging quality assurance systems

Overseas Certification
HKPC enhances enterprises’ product and service quality to fulfil international standards of production. Our service includes, but is not limited to:
- Current Good Manufacturing Practices (cGMPs) for Food and Dietary Supplements, FDA US
- NSF/ANSI Good Manufacturing Practice (GMP)
- Good Manufacturing Practice (GMP) for Medicines, TGA Australia
- Licence for Pharmaceutical Manufacturers (PIC/S GMP)
- Health Food Product Manufacturers (ISO 22000, HACCP)
- Good Manufacturing Practices for Cosmetics (ISO 22716)
- Medical Device - Quality Management System (ISO 13485)
Certification Application Assistance Service

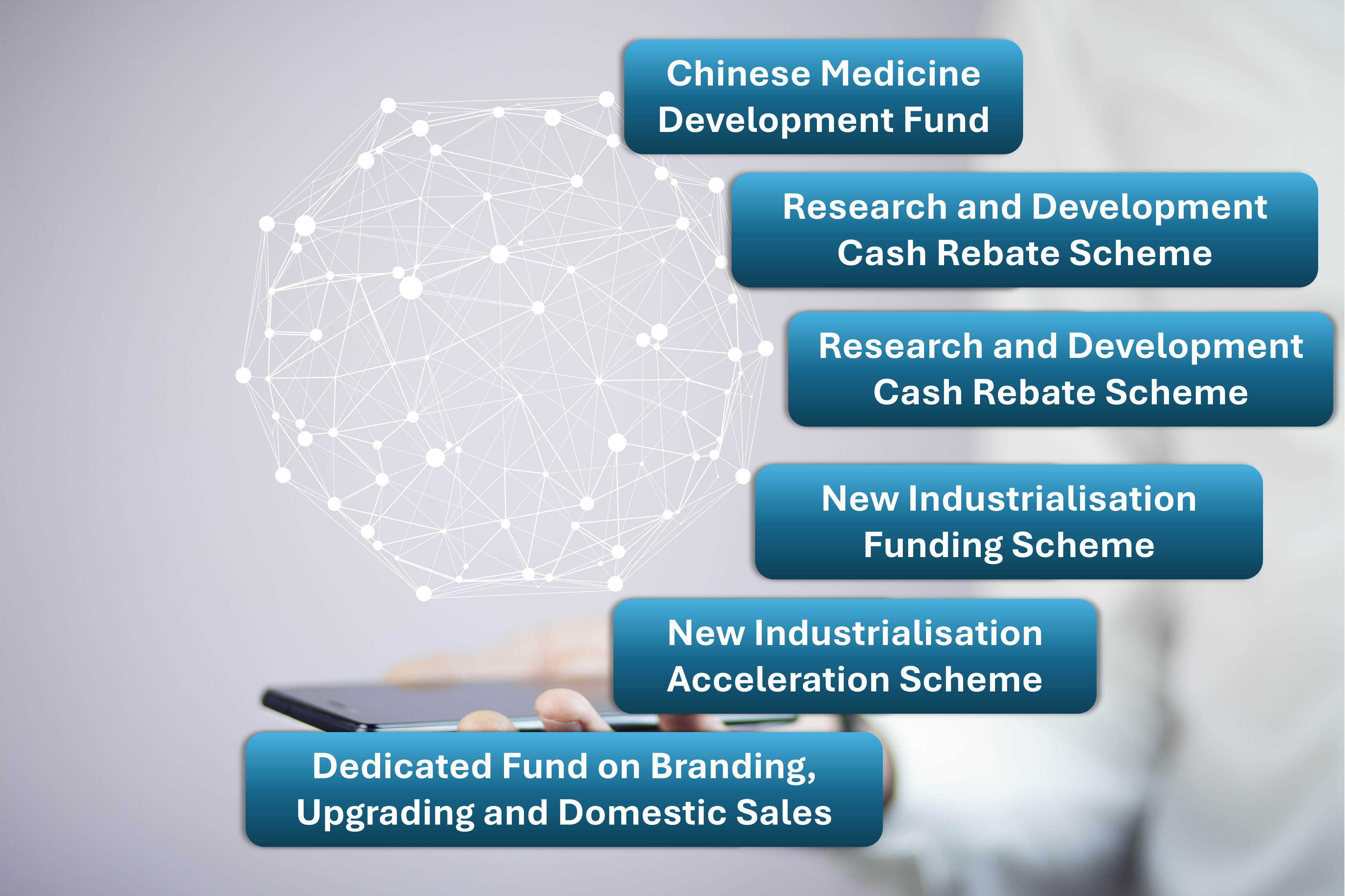
Funding and Policy Support
HKPC provides funding application support to local HealthTech and TCM enterprises, facilitating their international market expansion, including but not limited to:
- Chinese Medicine Development Fund (CMDF)
https://www.cmdevfund.hk/en/
- Research and Development Cash Rebate Scheme (CRS)
https://www.itf.gov.hk/en/crs
- Innovation and Technology Support Programme (ITSP)
https://www.itf.gov.hk/en/funding-programmes/supporting-research/itsp/itsp-platform-seed/index.html
- New Industrialisation Funding Scheme (NIFS)
https://www.itf.gov.hk/en/funding-programmes/facilitating-technology/nifs/
- New Industrialisation Acceleration Scheme (NIAS)
https://www.itf.gov.hk/en/funding-programmes/promoting-new-industrialisation/nias/index.html
- Dedicated Fund on Branding, Upgrading and Domestic Sales (BUD)
- Mainland Programme
https://mainland.bud.hkpc.org/en - FTA and IPPA Programme
https://fta.bud.hkpc.org/en
- Mainland Programme
Government funding programs
Share the latest information of HKPC to your inbox
Our Services
Support & Resource
HKPC Spotlights
COPYRIGHT© Hong Kong Productivity Council
